Remarkable solvent isotope dependence on gelation strength in low molecular weight hydro-gelators†
Abstract
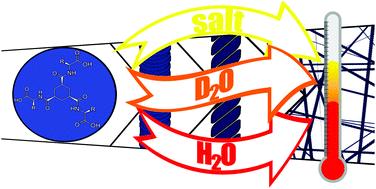
A delicate interplay of anisotropic hydrophobic/hydrophilic, π–π stacking, ionic and hydrogen bond interactions determine the strength of hydrogelators and are considered key factors in efforts to design potent small molecule hydrogelators. Here we show that solvent deuteration and electrolytic strength affect the strength of hydrogels formed from amino acid modified C3-symmetric cyclohexane trisamides profoundly. Gels formed by self-assembly through heating/cooling of solutions or by pH switching show up to a 30 °C increase in their melting temperatures in D2O compared to H2O. The unusually large solvent isotope effect on gel formation and thermal properties indicates that, in contrast to expectations, hydrogen bonding is not the primary determinant of gel strength but instead that hydrophobic interactions between the gelator molecules and the terminal carboxylic acid units are of greater importance. A conclusion that is supported by a similarly large effect of electrolytes on gel strength.


 Please wait while we load your content...
Please wait while we load your content...