Tertiary amine self-catalyzed intramolecular Csp3–H functionalization with in situ generated allenes for the formation of 3-alkenyl indolines†
Abstract
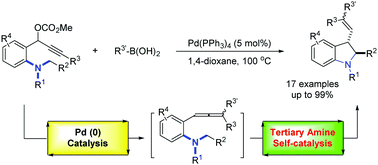
A novel methodology for the synthesis of 3-alkenyl indolines through the reaction of in situ generated allenes with N-benzylic Csp3–H has been developed. The reaction was realized by Pd(0) catalyzed allenylation of propargyl carbonate with organoboron and subsequent tertiary amine self-catalyzed Csp3–H functionalization in a one-pot process. Control reactions suggested that the substrate itself might also serve as a Lewis base for the N-benzylic Csp3–H functionalization.


 Please wait while we load your content...
Please wait while we load your content...