Photocyclization of photoswitches with high enantioselectivity in human serum albumin in an artificial environment†
Abstract
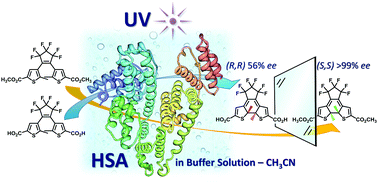
Three photochromic bisthienylethenes exhibited 56 to >99% enantiomeric excess in photochemical ring closure upon UV irradiation when incorporated in human serum albumin dissolved in 15% acetonitrile-phosphate buffer solution and incubated for 24 h at −4 °C. The absolute stereochemistry of the major enantiomers of two bisthienylethenes has been determined by their chemical transformations.


 Please wait while we load your content...
Please wait while we load your content...