Acid-catalysed thermal cycloreversion of a diarylethene: a potential way for triggered release of stored light energy?†
Abstract
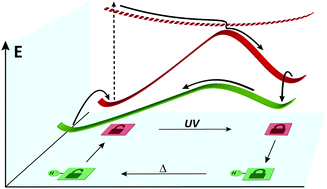
Upon addition of catalytic amounts of acid, a closed diarylethene derivative carrying a fluorenol moiety undergoes facile thermal ring opening. The underlying thermodynamics and kinetics of the entire system have been analysed experimentally as well as computationally. Our work suggests that general acid catalysis provides a useful tool to bypass thermal barriers, by opening new reaction pathways, and to efficiently trigger the release of light energy stored in photoswitches.


 Please wait while we load your content...
Please wait while we load your content...