Cooperative Lewis acid–onium salt catalysis as tool for the desymmetrization of meso-epoxides†
Abstract
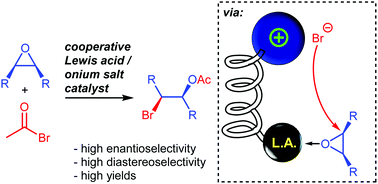
Epoxide desymmetrizations by bromide are very rare despite the large synthetic potential of chiral bromohydrins. Herein we present a new concept for epoxide desymmetrizations in which a bifunctional Lewis acid/ammonium salt catalyst allows for efficient enantioselective epoxide ring openings by Br−. With acetylbromide as a Br− source bromohydrin esters are formed.


 Please wait while we load your content...
Please wait while we load your content...