Synthesis of regioregular π-conjugated polymers consisting of a lactam moiety via direct heteroarylation polymerization†
Abstract
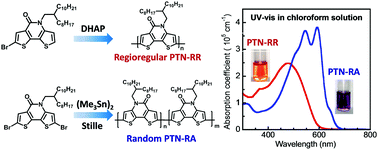
Regioregular polymers based on an asymmetric dithieno[3,2-b:2′,3′-d]pyridin-5(4H)-one (TN) unit that consists of a lactam moiety were synthesized via palladium-catalyzed direct heteroarylation polymerization. The random orientation of the lactam moiety can be prevented by carefully designing the monomers with tailored molecular structures. It is noted that connecting the TN unit in different fashions generates substructures in the polymer backbone with different electronic structures. Compared to the random counterparts, the regioregular homopolymers exhibit dramatically discrepant optical properties and electronic structures, while the variations in the copolymers are less distinguished.


 Please wait while we load your content...
Please wait while we load your content...