Synthesis of a fluorescent photoaffinity probe of OSW-1 by site-selective acylation of an inactive congener and biological evaluation†
Abstract
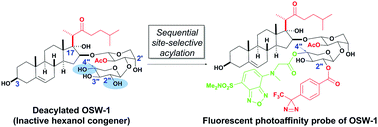
A novel fluorescent photoaffinity probe of OSW-1 was prepared in two steps from a naturally occurring inactive congener by a sequential site-selective acylation strategy using Me2SnCl2. It displayed highly potent anticancer activity and a similar intracellular localization property to that of a fluorescently-tagged OSW-1, thereby demonstrating its potential utility in live cell studies.
- This article is part of the themed collection: Site-selective molecular transformations

 Please wait while we load your content...
Please wait while we load your content...