Reactivity of the nitrogen-centered tryptophanyl radical in the catalysis by the radical SAM enzyme NosL†
Abstract
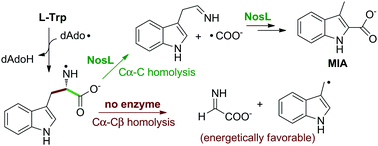
The radical SAM tryptophan (Trp) lyase NosL involved in nosiheptide biosynthesis catalyzes two parallel reactions, converting L-Trp to 3-methyl-2-indolic acid (MIA) and to dehydroglycine and 3-methylindole, respectively. The two parallel reactions diverge from a nitrogen-centered tryptophanyl radical intermediate. Here we report an investigation on the intrinsic reactivity of the tryptophanyl radical using a chemical model study and DFT calculations. The kinetics of the formation and fragmentation of this nitrogen-centered radical in NosL catalysis were also studied in detail. Our analysis explains the intriguing catalytic promiscuity of NosL and highlights the remarkable role this enzyme plays in achieving an energetically highly unfavorable transformation.


 Please wait while we load your content...
Please wait while we load your content...