Synthesis of oxindole from acetanilide via Ir(iii)-catalyzed C–H carbenoid functionalization†
Abstract
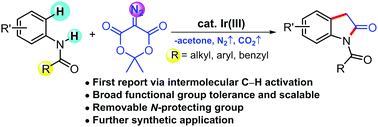
Herein we disclose the first report on the synthesis of oxindole derivatives from acetanilide via Ir(III)-catalyzed intermolecular C–H functionalization with diazotized Meldrum's acid. A broad range of substituted anilides were found to react smoothly under the Ir(III)-catalytic system to afford the corresponding N-protected oxindoles. The N-protecting groups, such as Ac, Bz or Piv, can be easily removed to furnish the oxindole. Various synthetic applications of the synthesized oxindole were also demonstrated.

 Please wait while we load your content...
Please wait while we load your content...