Gold(i)-catalyzed 6-endo-dig azide–yne cyclization: efficient access to 2H-1,3-oxazines†
Abstract
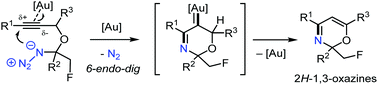
Denitrogenative 6-endo-dig azide–yne cyclization of α-propargyloxy-β-haloalkylazides was enabled by gold catalysis, thus providing 2H-1,3-oxazines. This rare cyclization mode in gold-catalyzed reactions of azide–yne substrates was demonstrated to be facilitated and controlled by electronic and resonance effects of the alkyne substituents. Molecular transformations of the as-prepared 2H-1,3-oxazines were also investigated.


 Please wait while we load your content...
Please wait while we load your content...