A simple design atomic emission spectrometer combined with multivariate image analysis for the determination of sodium content in urine†
Abstract
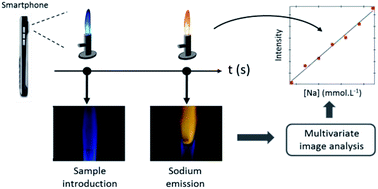
In clinical analysis the determination of alkali metals in biological samples is often performed by flame emission spectroscopy (FES). Despite its good analytical performance for the determination of trace elements, FES apparatus is often cumbersome, expensive, and energy dependent and thus, not applicable for on-site measurements. In an attempt to address these limitations, a simple digital atomic emission spectrometer built from easily available components is proposed in this study for the quantification of sodium in human urine samples. The system was designed to operate in the absence of electrical power supply with a sample consumption per measurement of about 10 μL. It comprises a traditional Bunsen burner with an air-propane flame embedded in a protective box, a silver wire loop for sample introduction and a smartphone camera for digital recording of the signal emitted by the analyte. The collected video frame is subjected to a multivariate image procedure based on multivariate curve resolution – alternating least squares (MCR-ALS) intended to avoid subjectivity in the selection of the region of interest (ROI) used to build the univariate regression model. The developed platform was evaluated in the context of sodium determination in human urine in the range from 40 to 220 mmol L−1. The system displays a linear response (R2 = 0.990) between the color index and sodium content for standard samples with a detection limit of 10.9 mmol L−1. Performance study for intra-day (2.5–4.1%) and inter-day (1.3–2.5%) measurements, performed on three different days, exhibits good repeatability. The homemade digital atomic emission spectrometer was successfully applied to the determination of (spiked) sodium in human urine samples (R2 = 0.942) with recovery that ranged from 94.8 to 110.4% and an averaged mean relative error below 10%. Selectivity study against potassium, calcium and magnesium revealed high tolerance of the method for these interferents.


 Please wait while we load your content...
Please wait while we load your content...