Practical corrections for p(H,D) measurements in mixed H2O/D2O biological buffers†
Abstract
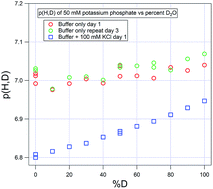
Mixtures of light and heavy water are used in NMR, small-angle neutron scattering (SANS), growth media for producing deuterated biological molecules, and analytical methods such as hydrogen–deuterium exchange (HDX) mass spectrometry. It is common to measure the pH of these solutions with a combination glass electrode with all chambers filled with aqueous (H2O) potassium chloride solutions. In the daily measurement of samples containing mixtures of H2O with D2O in some ratio – call this measurement p(H,D) – we generally do not control for all of the contributions to the differences measured in carefully controlled electrochemical experiments. For example, the calibration solutions contain relatively low concentrations of the calibrant buffer with low or no added salt. Meanwhile the tested solutions can contain widely varying levels of any number of different salts as well as both polar and nonpolar organics and polymers and proteins. In this note, the p(H,D) behaviors of 50 mM solutions of five different buffers used in biological in vitro solutions were measured over the full range of H2O : D2O ratios in the open atmosphere. After calibration, pH measurements were made with the buffer solutions alone and with 100 mM KCl added to model a significant ionic strength difference. The solutions consisted of 1 : 1 volume mixtures of the acid and base forms of acetate, monobasic/dibasic phosphate, 2-amino-2-hydroxymethyl-propane-1,3-diol (tris), 2-amino-2-hydroxymethyl-propane-1,3-diol (HEPES), and glycine to span the common, full range for biological buffers. The pH values of the 1 : 1 mixtures mean that the measurements were, in fact, of their formal pKa values. Each of the buffers exhibited a unique pattern of behavior, and none of them exhibited a measured ΔpKa = pKDa − pKHa as large as 0.4, a value that has been suggested to be added to a pH measured in H2O to match the equivalent pD measured in D2O. The results do indicate that when a reasonable, required accuracy for pH measurement is ±0.1 units, three general guidelines apply: (1) where p(H,D) values are less than 8 for any D2O content, no correction is needed for the p(H,D) measurement when comparing it to pHH; (2) for less than 50% D2O, if the 8 < p(H,D) < 10, again no correction is needed for the p(H,D) measurement compared to pHH; (3) when the D2O content is greater then 50% and the p(H,D) > 8, any corrections required will depend on the specific conditions and the specific buffer. Outside of the range 4 < p(H,D) < 10 or for needed greater accuracy, any corrections required will depend on the specific conditions and the identity of the buffer.


 Please wait while we load your content...
Please wait while we load your content...