The use of glutathione as a derivatising agent for the analysis of Michael acceptor genotoxic impurities in pharmaceuticals
Abstract
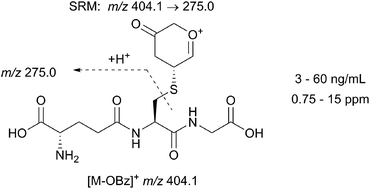
This work describes the development strategy and validation of an analytical method to determine trace levels of a genotoxic impurity, pyranone 2, in a pharmaceutical intermediate. A specific sensitive method capable of determining part per million (ppm) levels of this genotoxic impurity was required. The approach taken exploits the inherent Michael-acceptor reactivity present within the pyranone, utilising a novel derivatisation with glutathione followed by quantitative LC-MS/MS analysis or an LC-MS limit test. The methods have been demonstrated to be suitable to determine trace levels of the pyranone 2 at the required step in the API synthesis.


 Please wait while we load your content...
Please wait while we load your content...