Determination of Fe(iii) using digital images: study of corrosion in steel plates using a polyester laser printed device†
Abstract
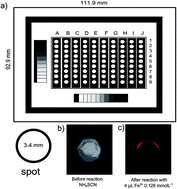
The marketing of metals and metal alloys requires strategies for controlling the integrity of these materials. Corrosion of iron alloys results in the formation of oxides on the metal surface and can be quantified in units of mass loss of the metal. In this article, a method was developed for determining the mass of iron oxide formed on the surface of metal using a polyester laser printed spot device. The technique involves the oxidation of Fe(II) to Fe(III) and a subsequent reaction of Fe3+ with SCN− to form a colored species. This reaction, which is widely used in chemical methods, employs reagents that are readily available and inexpensive. A template with 90 spots of diameter 3.4 mm was printed on a transparency sheet using a commercial laser printer. Each spot was impregnated with 4 μL of 0.190 mol L−1 ammonium thiocyanate solution. The reaction of Fe3+ with SCN− resulted in a product that absorbed in the visible region. Detection of the colored spots formed was performed automatically by acquisition of images with a scanner and a cell phone. The method developed was applied to the determination of corrosion rates in samples subjected to controlled corrosion processes. For comparison purposes, the corrosion was also determined by the gravimetric standard method. No significant differences between the results of both methods were observed, showing good performance with respect to the ASTM conventional reference method. The method developed offers advantages for routine analyses, with several spots that can be analyzed simultaneously and only a few units of μL of sample were required. Other advantages inherent in the method are drastic reduction in the use of reagents and the analytical cost, as well as waste generation.


 Please wait while we load your content...
Please wait while we load your content...