Rapid determination of the Mn average oxidation state of Mn oxides with a novel two-step colorimetric method
Abstract
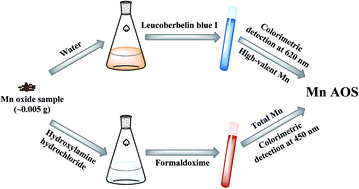
The Mn average oxidation state (Mn AOS) of Mn oxides has a significant impact on their reactivity towards trace metals and organic contaminants via sorption, catalysis and oxidation processes. Accurate determination of the Mn AOS is a key step to understanding the structures, composition, physicochemical properties, environmental behaviors and potential applications of Mn oxides. Here, a rapid two-step colorimetric method was developed to determine the Mn AOS of various Mn oxides, and it was tested on several Mn oxides (vernadite, acid birnessite and hausmannite). We also determined the Mn AOS of these Mn oxides with the conventional oxalic acid–permanganate back titration method and X-ray absorption near-edge spectroscopy (XANES) for comparison. In this rapid two-step colorimetric method, leucoberbelin blue I (LBB) and formaldoxime colorimetry were employed to obtain the oxidation numbers of high-valent Mn and total Mn, respectively, which were then used to calculate the Mn AOS. The colorimetric measurements are of considerable color stability and high sensitivity, thus enabling rapid, convenient and highly accurate determination of the Mn AOS compared with conventional methods. In addition, the required sample amount is greatly reduced (from ∼0.05 g to ∼0.005 g), making the proposed method an appropriate strategy for micro-volume samples.

 Please wait while we load your content...
Please wait while we load your content...