Acceleration of metal–ligand complexation kinetics by electrospray ionization†
Abstract
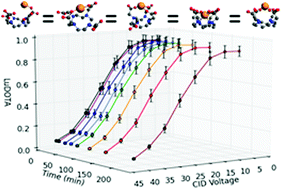
Electrospray ionization mass spectrometry (ESI-MS) is a valuable and frequently used analytical technique across nearly all branches of chemistry, and has recently seen increasing use in the study of metal–ligand solution equilibria. Despite its prevalence, the method by which ESI produces gas-phase ions from solutions containing metal–ligand complexes is not fully understood, with recent reports showing significant changes to solution equilibria during ESI analysis. This study examines perturbations to the formation kinetics of metal–ligand complexes during the ESI process, showing how quickly new equilibria – reflective of the ionization process and not solution – can be established. Electrospray ionization mass spectrometry (ESI-MS) and ion mobility spectrometry (IMS) are used to examine the well studied Lu-DOTA (1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid) complexation reaction, with collision cross section modeling based on density functional theory (DFT) optimized structures used to aid in the interpretation of the ion mobility results. The electrospray process was found to significantly accelerate the formation kinetics, increasing the formation rate constant by more than an order of magnitude over its previously determined solution-phase value.


 Please wait while we load your content...
Please wait while we load your content...