Characterization of lectin binding affinities via direct LC-MS profiling: implications for glycopeptide enrichment and separation strategies†
Abstract
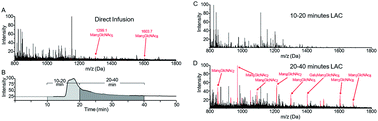
Determining the affinity between a lectin and its target glycans is an important goal, both for understanding the biological functions of a given lectin as well as enabling the use of that lectin for targeted enrichment of glycosylated species from complex samples. While the overall selectivities of many lectins have been characterized, such studies generally require individually purified lectins and glycans. From these analyses, it is clear that a given lectin does not bind all of its target glycans with the same affinity. Rather, lectins display a continuum of affinities for the range of glycan structures they may encounter. Because of this continuum, it is not straightforward in practice to determine which set of structures will be enriched using a lectin as an affinity reagent. Here we describe the development of glycan affinity chromatography coupled directly to electrospray mass spectrometry, which enables direct analysis of interactions of lectins with both glycans and glycoconjugates from complex mixtures. By observing the elution behavior of individual species, we are able to determine exactly which set of glycoconjugates would be enriched for a given lectin. Furthermore, this approach allows for the direct assessment of affinity constants between an individual lectin and a large number of glycans in a single experiment, which can be conducted using a complex mixture of unpurified glycans of varying concentrations.

 Please wait while we load your content...
Please wait while we load your content...