Fluorescent aryl naphthalene dicarboximides with large Stokes shifts and strong solvatochromism controlled by dynamics and molecular geometry†
Abstract
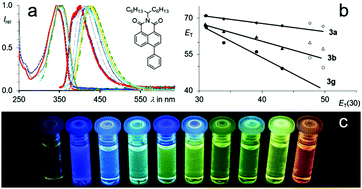
A series of highly fluorescent 4-aryl substituted naphthalene dicarboximides were efficiently prepared via metal organic C–C-coupling reactions. The obtained push–pull fluorophores display a distinct positive solvatochromism of the fluorescence. These optical properties are shown to be significantly dependant on the molecular geometry. Corresponding to TICT, a twist between the donor and the acceptor moiety enhances the intramolecular charge transfer resulting in such pronounced solvatochromism. Complete orthogonalisation inhibits the fluorescence. An intentional skew arrangement leads to solvent-adjustable chromophores with high fluorescence quantum yields and Stokes shifts of more than 1.6 eV.

 Please wait while we load your content...
Please wait while we load your content...