Quinoxaline-based cross-conjugated luminophores: charge transfer, piezofluorochromic, and sensing properties†
Abstract
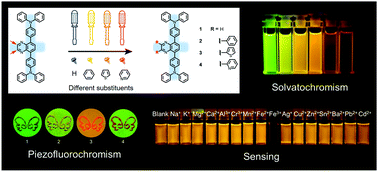
A series of symmetrical cross-conjugated luminophores with different substituents at the 2,3-positions of a quinoxaline unit have been designed and synthesized. Their intramolecular charge transfer (ICT), piezofluorochromic (PFC), and sensing properties have been systematically investigated. It can be found that four luminophores with the same conjugation backbone exhibit different ICT interactions between the electron-withdrawing quinoxaline unit and electron-donating triphenylamine units. Lippert–Mataga analysis reveals that luminophore 1 without substituents displays the largest dipole moment difference between ground and excited states. Moreover, the target luminophores present reversible PFC properties due to the phase transitions between crystalline and amorphous states. The most remarkable piezofluorochromism is achieved for luminophore 4 with pyridyl substituents. A bathochromic shift of 40 nm can be observed upon grinding the pristine sample. Furthermore, the target luminophores demonstrate selective and sensitive sensing properties to Fe3+ ions. In addition, luminophore 4 can also act as a colorimetric and fluorescent chemosensor for Ag+ ions.

 Please wait while we load your content...
Please wait while we load your content...