Geometrically isomeric Pt(ii)/Fe(ii)-based heterometallo-supramolecular polymers with organometallic ligands for electrochromism and the electrochemical switching of Raman scattering†
Abstract
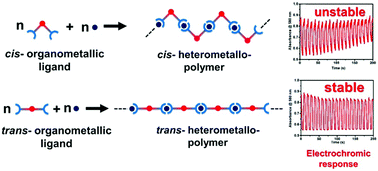
Heterometallo-supramolecular polymers with Pt(II) and Fe(II) ions introduced alternately (cis-polyPtFe and trans-polyPtFe) in a precise way were prepared successfully by the 1 : 1 complexation of Fe(II) ions with cis- or trans-conformational organo-Pt(II) ligands. The conformational difference between cis- and trans-greatly changed the morphology, crystallinity, ionic conductivity, electrochromic properties, and redox-triggered fluorescence of the polymers. The cis-polyPtFe exhibited better crystallinity and low ionic conductivity, whereas trans-polyPtFe showed an amorphous nature with high ionic conductivity. Both the polymers exhibited reversible electrochromism between purple and yellow colors due to the redox of Fe(II)/(III) upon applying a potential of 0 V or +3 V. The trans-polyPtFe showed better electrochromic stability and response times compared to cis-polyPtFe. In addition, the trans-polyPtFe also showed an improved response in redox-triggered Raman scattering switching compared to cis-polyPtFe over a long time range.

 Please wait while we load your content...
Please wait while we load your content...