Clarifying the preferential occupation of Ga3+ ions in YAG:Ce,Ga nanocrystals with various Ga3+-doping concentrations by nuclear magnetic resonance spectroscopy†
Abstract
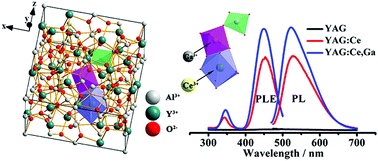
As candidates for display and lighting optical materials, yttrium aluminum garnet nanophosphors co-doped with Ce3+ and Ga3+ ions (YAG:Ce,Ga) were successfully synthesized via a facile sol–gel method. We used multiple characterization techniques to study the local crystal structure of Ce3+ activators and examine the preferential position of Ga3+ ions in YAG:Ce,Ga nanophosphors. For the first time, solid-state nuclear magnetic resonance spectroscopy was used to confirm that Ga3+ ions preferentially replaced the octahedral Al3+ ions in a wide Ga3+ concentration range. The replacement caused the expansion of the lattice constant and decreased the local crystal field strength surrounding Ce3+ activators that located in the dodecahedral sites, which were beneficial to the luminescence emission of Ce3+ ions. The YAG:Ce,Ga nanophosphors with excellent photoluminescence properties were obtained by optimizing the calcination temperature (930 °C) and Ga3+-doping concentration (18 at%). The optimized nanophosphors displayed stronger luminescence emission with a desirable blue shift than classic YAG:Ce phosphors, and presented a short fluorescence lifetime of ∼24 ns and a long fluorescence lifetime of ∼85 ns.

 Please wait while we load your content...
Please wait while we load your content...