Aromatic azaheterocycle-cored luminogens with tunable physical properties via nitrogen atoms for sensing strong acids†
Abstract
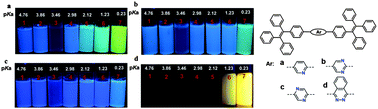
A series of luminogens comprising one pyridine, 1,3-diazine, 1,4-diazine, 1,2-diazine and phthalazine moiety as the central core and two AIE-active tetraphenylethene units in lateral positions have been readily synthesized by Suzuki cross-coupling. They exhibited remarkably different photophysical and electrochemical properties, as well as solid packing and fine controllability via the number and position of the nitrogen atoms in the aromatic azaheterocycle core. Among them, the pyridine, 1,3-diazine and 1,4-diazine-cored luminogens displayed strong AIE activities, whereas the 1,2-diazine and phthalazine-cored luminogens exhibited almost no AIE effect. The intrinsic Lewis basicity of the as-prepared luminogens endowed them with the ability to fluorometrically detect acids with different pKa values. When protonated by a strong acid such as trifluoroacetic acid, the pyridine, 1,3-diazine and 1,4-diazine-cored luminogens displayed relatively weak AIE effects. In contrast, the 1,2-diazine and phthalazine-cored luminogens exhibited highly sensitive responses to strong acids within a precise pKa range by displaying turn-on fluorescence emissions in the low-energy region, which was probably owing to the synergetic effect of AIE and the constraint of the intersystem crossing effect upon protonation of the 1,2-diazine segment. They displayed reversible acidochromism in response to protonation and deprotonation in the solid state. Such unique properties of the as-prepared luminogens could be used for the selective discrimination of some organic acids, which is highly valuable in the study of biological metabolism.

 Please wait while we load your content...
Please wait while we load your content...