A new approach to polycyclic azaarenes: visible-light photolysis of vinyl azides in the synthesis of diazabenzopyrene and diazaperylene†
Abstract
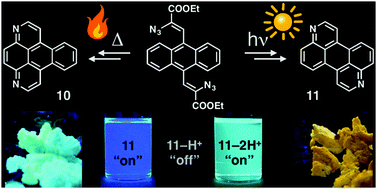
Nitrogen containing polycyclic aromatics are attractive π-functional materials due to their stability and interesting electronic and optical properties. As such, new avenues for the incorporation of nitrogen heteroatoms into arenes are desirable. The visible-light photocyclization of vinyl azides is an efficient method to form nitrogen heterocycles, but previously required complex photocatalysts. Herein we report the cyclization of vinyl azide derivatives of anthracene via either thermal activation or visible-light activation without a photocatalyst. The resulting products are two novel azaarenes: 1,8-diazabenzo[e]pyrene and 3,9-diazaperylene. The electrochemical and photophysical properties of the materials are compared to those of their hydrocarbon analogues. Both materials exhibit increased electron affinities and high quantum yields (up to 82%). Successive protonation of the nitrogen heteroatoms first quenches, then fully restores fluorescence at longer wavelengths, indicating possible applications as pH sensors.

 Please wait while we load your content...
Please wait while we load your content...