Star-shaped D–π–A oligothiophenes with a tris(2-methoxyphenyl)amine core and alkyldicyanovinyl groups: synthesis and physical and photovoltaic properties†
Abstract
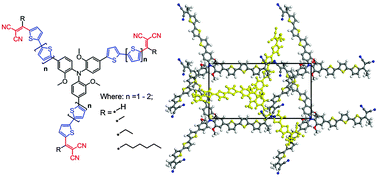
Synthesis of a series of star-shaped oligomers having a novel electron donating tris(2-methoxyphenyl)amine (m-TPA) core, which is linked through a bithiophene or terthiophene π-bridge with electron-deficient alkyldicyanovinyl (alkyl-DCV) groups, is described. A comprehensive study of the oligomers revealed significant dependence of their physical properties, including absorption, molecular frontier energy levels, crystal packing, and melting and glass transition temperatures, upon the chemical structure. A comparison of their photophysical properties to the nearest analog having the common dicyanovinyl (DCV) groups demonstrated a number of benefits to use alkyl-DCV units for the design of donor–acceptor small molecules: higher solubility, increased electrochemical stability, better photovoltaic performance, and possibility to control the relative physical and photovoltaic properties by a simple adjustment of alkyl and π-bridge lengths. Modification of the well-known triphenylamine (TPA) core in the star-shaped oligomers by methoxy groups increases not only solubility, but also crystallinity of the oligomers, whereas their photovoltaic performance stays on a similar level as their analogs with a TPA core. The study demonstrates that these design strategies represent interesting and simple tools for the effective modulation of properties of star-shaped molecules.


 Please wait while we load your content...
Please wait while we load your content...