Towards solution processable air stable p-type organic semiconductors: synthesis and evaluation of mono and di-fluorinated pentacene derivatives†
Abstract
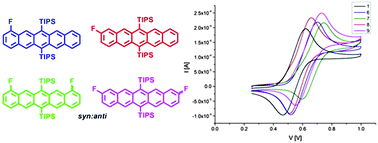
The synthesis of a series of mono and di-fluorinated TIPS pentacene compounds for application as organic semiconductors is reported. Namely the novel 1-fluoro-6,13-bis[triisopropylsilyl-ethynyl]pentacene (1-fluoro-TIPS-pentacene), 2-fluoro-TIPS-pentacene and the recently reported 1 : 1 syn : anti isomeric mixtures of 1,(8 : 11)-difluoro-TIPS-pentacenes and 2,(9 : 10)-difluoro-TIPS-pentacenes. For the first time these materials have been subjected to a study of the relationship between molecular structure and thermal/photochemical stability. The changes in the pentacene HOMO via alteration of fluorine substitution is studied and related to environmental stability. As predicted, it was found that increasing the number of electron-withdrawing fluorine substituents increases stability to photodegradation. It was also observed that stability increases (and HOMO decreases) when fluorine is present on the 1- and 1,(8 : 11)-positions closer to the central rings rather than in the 2- and 2,(9 : 10)-positions. Comparative transistor measurements for the fluoro-derivatives demonstrated competitive device performance in comparison to underivatised TIPS-pentacene. Full characterisation and NMR assignment of these materials has been accomplished by comparison over the series for the first time.

 Please wait while we load your content...
Please wait while we load your content...