Perovskite ferroelectrics and relaxor-ferroelectric solid solutions with large intrinsic electrocaloric response over broad temperature ranges
Abstract
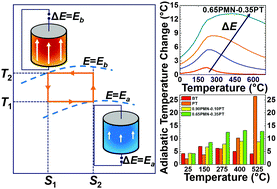
Electrocaloric materials have emerged as a viable technology for solid state heating/cooling and waste heat recovery applications. We determine here intrinsic electrocaloric (EC) entropy and temperature changes in perovskite ferroelectrics (FEs) using theoretical tools supported by experimentally measured heat capacities as a function of the applied electric field and temperature. A quantitative theoretical analysis of the thermal, pyroelectric and EC properties of representative ceramic FE systems is provided for BaTiO3 and PbTiO3 (PT) that display a weak and a strong first-order phase transformation to the FE state, and relaxor-FE xPb(Mgl/3Nb2/3)O3–(1 − x)PbTiO3 [xPMN−(1 − x)PT] solid solutions. Our results indicate that the intrinsic adiabatic temperature changes in relaxor-FEs are substantial. Temperature variations (related to reduced entropy) as high as 14 °C can be achieved with applied fields on the order of 1 MV cm−1 at T ∼ 350 °C for (001) oriented 0.65PMN–0.35PT. Moreover, the EC response does not vary over a large temperature interval: 13 ± 1 °C for 200 < T < 600 °C. In PT, the adiabatic temperature change is approximately 28 °C for an applied field of 1 MV cm−1, rivaling the best EC response observed in polymer FEs. This study provides the general methodology for theoretical analysis to both assess and guide the way in discovering newer high performance EC materials.

 Please wait while we load your content...
Please wait while we load your content...