Highly efficient green phosphorescent organic light-emitting diodes with low efficiency roll-off based on iridium(iii) complexes bearing oxadiazol-substituted amide ligands†
Abstract
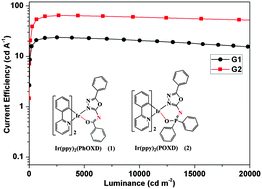
Two novel iridium(III) complexes [Ir(ppy)2(PhOXD)] (1, ppy = 2-phenylpyridine, PhOXD = N-(5-phenyl-1,3,4-oxadiazol-2-yl)-benzamide) and [Ir(ppy)2(POXD)] (2, POXD = N-(5-phenyl-1,3,4-oxadiazol-2-yl)-diphenylphosphinic amide) have been designed and synthesized, and their photoluminescence and electrochemistry properties were investigated. At room temperature, complexes 1 and 2 show green emissions at about 502 and 506 nm with photoluminescence quantum yields (PLQYs) of 0.03 and 0.05 in CH2Cl2 solutions, respectively. In the 5 wt% doped poly(methyl methacrylate) (PMMA) film, the PLQYs (0.42 for complex 1, 0.52 for complex 2, respectively) increase significantly. The organic light emitting diodes (OLEDs) with the structure of ITO/HAT-CN (dipyrazino[2,3-f:2′,3′-h]quinoxaline-2,3,6,7,10,11-hexacarbonitrile), 10 nm)/TAPC (1,1-bis[4-(di-p-tolylamino)phenyl]cyclohexane, 40 nm)/Ir complexes (10 wt%): mCP (1,3-bis(9H-carbazol-9-yl)benzene, 20 nm)/TPBi (1,3,5-tris(1-phenyl-1H-benzimidazol-2-yl)benzene, 40 nm)/LiF (1 nm)/Al (100 nm) show good performances. In particular, device G2 based on complex 2 shows superior performances with a peak current efficiency (ηc) of 64.7 cd A−1 and a peak power efficiency (ηp) of 42.5 lm W−1 with low electroluminescence efficiency roll-off. The ηc value still remains over 60.6 cd A−1 even at a luminance of 10 000 cd m−2, which indicates that introducing electron transporting 1,3,4-oxadiazole and diphenyl phosphoryl groups into iridium complexes is an effective means of achieving efficient phosphors in OLEDs.

 Please wait while we load your content...
Please wait while we load your content...