Heterovalent Pb-substitution in ferroelectric bismuth silicate Bi2SiO5
Abstract
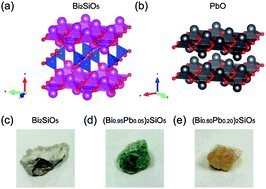
Systematic tuning of the ferroelectric phase transition in Bi2SiO5 was demonstrated using element substitution, where nominally heterovalent Pb was successfully substituted for Bi up to 20%. Comprehensive studies using powder X-ray diffraction analysis, dielectric measurements, X-ray absorption spectroscopy, and first-principles calculations revealed that charge compensation for heterovalent substitution was achieved via introduction of a considerable number of oxygen vacancies in Bi2O2 layers, resulting in the nominal composition (Bi1−x3+Pbx2+)2SiO5−x. The oxygen vacancies were found to induce local disordering of SiO4 tetrahedral chains, sandwiched between the Bi2O2 layers, leading to a decrease in the ferroelectric phase transition temperature and broadening of the phase transition in Bi2SiO5.

 Please wait while we load your content...
Please wait while we load your content...