Effects of pyridyl group orientations on the optoelectronic properties of regio-isomeric diketopyrrolopyrrole based π-conjugated polymers†
Abstract
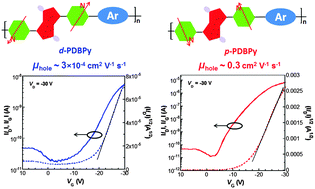
Two novel regio-isomeric π-conjugated polymers consisting of the pyridyl unit flanked by diketopyrrolopyrrole as the electron-accepting unit and 2,5-bis(3-hexylthiophen-2-yl)thieno[3,2-b]thiophene as the electron-donating unit were designed and synthesized. The comparison of the optical and electrochemical properties indicated that the copolymer based on the nitrogen atom proximal to the central diketopyrrolopyrrole unit (p-PDBPy) exhibited bathochromic shifted absorption spectra and narrower bandgaps than the counterpart copolymer (d-PDBPy) with the distally oriented nitrogen atom, which can be correlated with the stronger intermolecular aggregation of the former as a result of the different intrinsic molecular geometry of the polymer backbone. Of particular interest is that the copolymer p-PDBPy exhibited a moderate hole mobility of 0.35 cm2 V−1 s−1, which is about three orders of magnitude higher than the hole mobility of 3.2 × 10−4 cm2 V−1 s−1 obtained based on the counterpart copolymer d-PDBPy, as measured using organic field-effect transistors. These results demonstrated that the delicate control of the pyridyl orientations along the polymer backbone is of vital importance for the molecular design of π-conjugated polymers for high-performance organic electronic devices.

 Please wait while we load your content...
Please wait while we load your content...