BN-heteroacene-cored luminogens with dual channel detection for fluoride anions†
Abstract
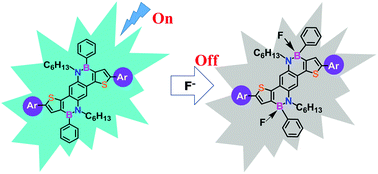
Very recently, polycyclic aromatic hydrocarbons (PAHs) have been extremely extended by replacing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C units with isoelectronic B–N ones, however, BN-containing π-conjugated oligomers or polymers are still very less explored due to the lack of appropriate building blocks. Herein, on the basis of the successful synthesis of a new BN-embedded heteroacene and its brominated derivatives, a series of BN-containing oligomers have been achieved via Suzuki cross-coupling with aryl boronic esters. Their rich photophysical properties and electrochemical behaviors are essentially dependent on the main chain lengths, indicative of the fully-π-conjugated effect of such kinds of luminogens. Furthermore, these BN-containing luminogens enable colorimetric and fluorometric dual channel detection of fluoride ions through binding to the Lewis acid boron atom of the BN moiety in high selectivity and sensitivity.
C units with isoelectronic B–N ones, however, BN-containing π-conjugated oligomers or polymers are still very less explored due to the lack of appropriate building blocks. Herein, on the basis of the successful synthesis of a new BN-embedded heteroacene and its brominated derivatives, a series of BN-containing oligomers have been achieved via Suzuki cross-coupling with aryl boronic esters. Their rich photophysical properties and electrochemical behaviors are essentially dependent on the main chain lengths, indicative of the fully-π-conjugated effect of such kinds of luminogens. Furthermore, these BN-containing luminogens enable colorimetric and fluorometric dual channel detection of fluoride ions through binding to the Lewis acid boron atom of the BN moiety in high selectivity and sensitivity.

 Please wait while we load your content...
Please wait while we load your content...