The morphology and structure of vanadyl phthalocyanine thin films on lithium niobate single crystals†
Abstract
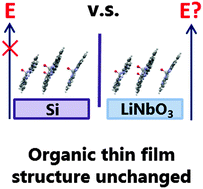
The electric field of ferroelectric materials has been used as a driving force to promote molecular adsorption and control the orientation of small dipolar molecules. This approach has not been investigated on larger polyaromatic molecules, such as those used in organic electronic devices, even though the physical and electronic properties of thin films are strongly dependent on molecular structure and orientation, ultimately affecting device performance. Here we investigate the effects of model ferroelectric surfaces on a dipolar organic semiconducting molecule. Thin films of vanadyl phthalocyanine (VOPc) deposited on to (0001) and (2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) lithium niobate were subjected to structural and morphological analysis. Whilst thin films could be grown on these surfaces, no obvious change to their structure or morphology was observed suggesting there was no influence of a surface electrical field or surface chemistry on the film structure, and that the substrate is more complex than previously thought.
0) lithium niobate were subjected to structural and morphological analysis. Whilst thin films could be grown on these surfaces, no obvious change to their structure or morphology was observed suggesting there was no influence of a surface electrical field or surface chemistry on the film structure, and that the substrate is more complex than previously thought.


 Please wait while we load your content...
Please wait while we load your content...