Solid solution between lithium-rich yttrium and europium molybdate as new efficient red-emitting phosphors†
Abstract
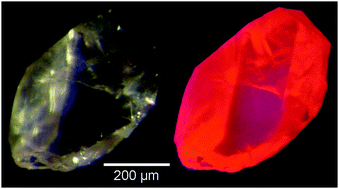
Li3.5Y1.5(MoO4)4 was synthesized in the form of phase-pure crystals. It forms a solid solution with Li3.5Eu1.5(MoO4)4. Single crystals obtained from Li2MoO4 fluxes had edge lengths of up to 0.5 mm. The crystal structures of Li3.5(Y1−xEux)1.5(MoO4)4 (x = 0, 0.1, 0.25, 0.5, 0.75 and 1) were determined in the triclinic crystal system (e.g. P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , no. 2, Z = 1; Li3.5Y1.5(MoO4)4: a = 5.1875(2) Å, b = 6.6380(2) Å, c = 10.2731(4) Å, α = 100.082(3)°, β = 100.257(2)° and γ = 111.943(2)°). All the compounds are isostructural and crystallize with a structure related to the scheelite-type structure with mixed occupancy of one cation position, while the two others are occupied by Li ions exclusively. Thermal analyses reveal stability in air up to 995 K for Li3.5Y1.5(MoO4)4; the decomposition temperature decreases with an increase in Eu-content. Spectroscopic properties (emission and excitation) were investigated in the context of the search for new thermographic phosphors. Excitation at 395 nm leads to strong red emission at 613 nm. Features of the emission spectra suggest the potential of Li3.5(Y1−xEux)1.5(MoO4)4 as red phosphor candidates for light emitting diodes. Their luminescence properties at high temperatures were also investigated.
, no. 2, Z = 1; Li3.5Y1.5(MoO4)4: a = 5.1875(2) Å, b = 6.6380(2) Å, c = 10.2731(4) Å, α = 100.082(3)°, β = 100.257(2)° and γ = 111.943(2)°). All the compounds are isostructural and crystallize with a structure related to the scheelite-type structure with mixed occupancy of one cation position, while the two others are occupied by Li ions exclusively. Thermal analyses reveal stability in air up to 995 K for Li3.5Y1.5(MoO4)4; the decomposition temperature decreases with an increase in Eu-content. Spectroscopic properties (emission and excitation) were investigated in the context of the search for new thermographic phosphors. Excitation at 395 nm leads to strong red emission at 613 nm. Features of the emission spectra suggest the potential of Li3.5(Y1−xEux)1.5(MoO4)4 as red phosphor candidates for light emitting diodes. Their luminescence properties at high temperatures were also investigated.

 Please wait while we load your content...
Please wait while we load your content...