Micelle-forming HPMA copolymer conjugates of ritonavir bound via a pH-sensitive spacer with improved cellular uptake designed for enhanced tumor accumulation
Abstract
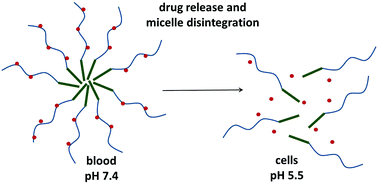
We describe design, synthesis, physico-chemical characterization and preliminary biological evaluation of micelle-forming polymer drug conjugates with controlled drug release intended for tumor treatment. The structure of the conjugates was designed to enable tumor tissue- and cell-specific drug release and micelle disassembly to avoid side effects accompanying classic chemotherapy and guarantee safe elimination of the drug-free carrier from the organisms. The amphiphilic polymer conjugates consisted of a hydrophobic hexaleucine block and a hydrophilic block based on the N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer with an antiviral and cytostatic drug, ritonavir, bound through a pH-sensitive spacer. Diblock copolymers with low dispersity (Đ ∼ 1.1) were prepared via reversible addition-fragmentation chain transfer (RAFT) copolymerization using a hexaleucine derivative as a chain transfer agent. The associative properties of the copolymers depend on the hydrophilic polymer block length and the hydrophobic ritonavir content. The micelles dissociated under mild acidic conditions mimicking the environment inside tumor tissue/cells, because of the decrease in polymer hydrophobicity after the rapid release of the hydrophobic drug from the polymer carrier. Unexpectedly, the polymer–ritonavir conjugates internalized into HeLa cells significantly more than the polymers without ritonavir. The enhanced cell penetration and pH-triggered micelle disassembly predetermine the polymer–ritonavir conjugates to become promising tumor-targeted drug carriers.

 Please wait while we load your content...
Please wait while we load your content...