Near-infrared fluorescence of π-conjugation extended benzothiazole and its application for biothiol imaging in living cells†
Abstract
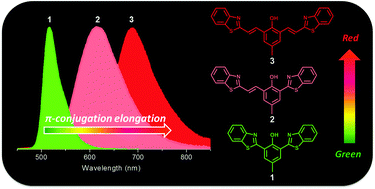
Two new π-conjugation extended benzothiazole derivatives bearing single (2) or double (3) vinyl groups between benzothiazolyl and 4-methylphenol moieties in 2,6-dibenzothiazolyl-4-methylphenol (1) have been synthesized and their photophysical properties were studied. In nonpolar solvents, 2 showed a keto tautomer emission from the excited state intramolecular proton transfer (ESIPT) at 626 nm, remarkably red-shifted compared to the keto tautomer fluorescence of 1 (568 nm), whereas 3 only exhibited an enol emission at 420 nm. However, a near-infrared (NIR) emission at 616/688 nm for 2/3 from the deprotonated anionic species was observed in polar solvents, which is farther red-shifted compared to that for deprotonated 1 (516 nm). With the aid of computational studies, the experimental observations were rationalized according to the efficient extension of π-conjugation of the molecular backbones in 2 and 3. Furthermore, a white emission with a broad band between 400 and 800 nm from 3 was also observed in a polar–nonpolar solvent mixture, where nearly pure white coordinates (0.33, 0.35) from the CIE chromaticity diagram were successfully achieved. By masking the phenol group in 3, a NIR fluorescent probe (4) for biothiols was constructed, which allows for imaging applications in living cells.

 Please wait while we load your content...
Please wait while we load your content...