Accentuated transdermal application of glucosamine sulphate attenuates experimental osteoarthritis induced by monosodium iodoacetate†
Abstract
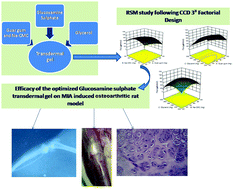
Osteoarthritis is a chronic degenerative joint disease causing pain and disability. Glucosamine sulphate is a well known oral supplement for its treatment. The present pioneering study provides an overview of the accentuated transdermal delivery of glucosamine sulphate through the optimized gel formulation with guar gum and sodium carboxymethyl cellulose (Na-CMC). Response surface methodology based on the three-level three-factor central composite design provided the optimum concentration of guar gum, Na-CMC and glycerol for a maximum flux. The transdermal characterization, ex vivo permeation study and in vivo study were performed with optimized gel formulation. The factorial design predicted the optimum values of guar gum, Na-CMC and glycerol which were 418.53 mg, 444.97 mg and 2322.4 mg respectively for 25 g of the gel. This optimized gel demonstrated the maximum flux, i.e., 1047.46 μg cm−2 h−1. The optimized gel showed satisfactory results with respect to drug uniformity, pH, stability, rheological properties, zeta potential, drug–excipient compatibility and skin irritation. The release of the drug from the optimized transdermal gel followed the controlled first order Fickian (non-steady) release pattern. The in vivo study was carried out in a rat model of osteoarthritis induced by monosodium iodoacetate damaging the tibial plateau. In this study the optimized formulation effectively reduced the symptoms like reduction in swelling of the knee joint, gross changes in digitized radio images and morphological and histopathological alterations. Additionally the changes in the release pattern of the proinflammatory cytokine tumor necrosis factor-α illustrated the efficacy of the transdermal gel for the treatment of experimental osteoarthritis. Thus the optimized gel was found to be a unique potential vehicle for transdermal application of glucosamine sulphate which effectively attenuates the experimental osteoarthritis.

 Please wait while we load your content...
Please wait while we load your content...