Tunable mesoporous manganese oxide for high performance oxygen reduction and evolution reactions†
Abstract
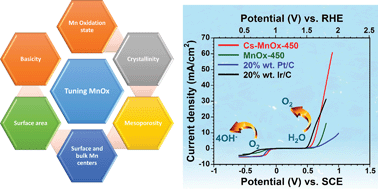
Understanding the origin of manganese oxide activity for oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) is a key step towards rationally designing of highly active catalysts capable of competing with the widely used, state-of-art noble metal catalysts. Herein, we present a bifunctional, thermally stable cesium-promoted mesoporous manganese oxide (Cs-MnOx) tuned by simple heat treatment from an amorphous to a crystalline phase with controlled surface and bulk active Mn centers. The Cs-MnOx material exhibited the highest ORR activity (0.87 V vs. RHE at −3 mA cm−2) among all noble-metal-free manganese oxide catalysts reported to date with superior activity compared to state-of-the-art Pt/C catalyst. In addition, Cs-MnOx exhibited comparable OER performance with the highly active Ir/C and RuO2 catalysts. Extensive characterization and density functional theory (DFT) computations suggested that the stabilization of the surface and bulk enriched Mn3+ species, increase of relative basicity and maintaining active crystalline phase due to Cs incorporation, are the main decisive factors for the profound ORR and OER activities. Findings from our study provide general guidance for designing of cost effective and active metal oxide based electrocatalysts.

 Please wait while we load your content...
Please wait while we load your content...