Unprecedented adsorptive removal of Cr2O72− and methyl orange by using a low surface area organosilica†
Abstract
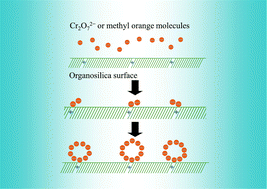
An extraordinary adsorption capacity of 359 and 1679 mg g−1 for the adsorptive removal of Cr2O72− and methyl orange (MO), respectively, was observed by using a low surface area (SABET of 10 m2 g−1) organosilica. The organosilica samples were synthesized by condensing phosphonitrilic chloride trimer with N1-3-(trimethoxysilylpropyl)ethylenetriamine followed by hydrolysis and co-condensation with different amounts of tetraethyl orthosilicate (TEOS). The kinetics, isotherms and thermodynamics of the adsorption process were investigated. The very high adsorption capacity of the low surface area adsorbent could be attributed to the formation of hemimicelles at the adsorbed sites of the adsorbent which suddenly increases the adsorption rate. An isotherm model was proposed similar to the adsorption model reported by Zhu and Gu with some modification. A pseudo-second order kinetic model fits best with the experimental data. Thermodynamically, as expected the present adsorption process is spontaneous and exothermic.

 Please wait while we load your content...
Please wait while we load your content...