β-Cyclodextrin modified graphitic carbon nitride for the removal of pollutants from aqueous solution: experimental and theoretical calculation study†
Abstract
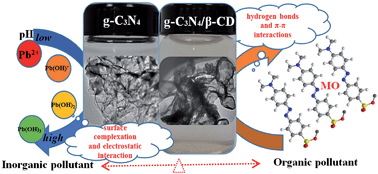
A novel β-cyclodextrin modified, multifunctional, layer-by-layer graphitic carbon nitride (g-C3N4/β-CD) was successfully synthesized and applied as an effective adsorbent for the removal of methyl orange (MO) and Pb(II) from aqueous solutions under various environmental conditions (e.g., solution pH, solid content, contact time and temperature). The kinetic results indicated that the adsorption was dominated by chemisorption, and the higher adsorption capacity of g-C3N4/β-CD was attributed to it having more oxygen-containing functional groups than g-C3N4. The Langmuir, Freundlich and Sips models were applied to simulate the adsorption isotherms of MO and Pb(II), and the results demonstrated that the adsorption of MO was attributed to multilayer adsorption, while the coverage adsorption of Pb(II) on the g-C3N4/β-CD was monolayer adsorption. The thermodynamic parameters showed that the adsorption of both MO and Pb(II) was spontaneous and endothermic. The DFT calculations further evidenced the surface complexation and electrostatic interaction of Pb(II) on the g-C3N4 and g-C3N4/β-CD, whereas, the interaction of MO with g-C3N4 and g-C3N4/β-CD was mainly attributed to hydrogen bonds and strong π–π interactions. The results demonstrated that g-C3N4/β-CD is a promising material for the efficient removal of organic and inorganic pollutants in environmental pollution remediation.

 Please wait while we load your content...
Please wait while we load your content...