Impact of the alkyl side chain position on the photovoltaic properties of solution-processable organic molecule donor materials†
Abstract
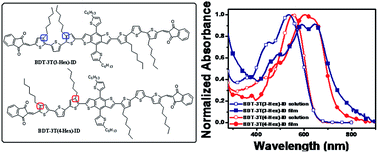
Two new A–D–A structured organic molecules with bithienyl-substituted benzodithiophene (BDT) as the core and donor unit, indenedione (ID) as the end group and acceptor unit, 3,3′′-dihexyl-2,2′:5′,2′′-terthiophene (3T(3-Hex)) or 4,4′′-dihexyl-2,2′:5′,2′′-terthiophene (3T(4-Hex)) as the π bridge, BDT-3T(3-Hex)-ID and BDT-3T(4-Hex)-ID, were designed and synthesized. The two compounds with the alkyl side chains at different positions in the π bridge backbone which are applied in solution-processable organic solar cells (OSCs) as donor materials have the same molecular weight and a similar structure, but exhibit different optical and photovoltaic properties. The BDT-3T(4-Hex)-ID film shows a broad absorption band from 400 nm to 750 nm with an absorption peak about 20 nm red-shifted compared to that of BDT-3T(3-Hex)-ID in solution, benefitting from the outward alkyl side chain in its structure. The power conversion efficiency (PCE) of the solution-processed OSC based on a blend of BDT-3T(4-Hex)-ID and PC71BM (1.25 : 1, w/w) reached 6.55% with a Jsc of 10.54 mA cm−2, a Voc of 0.87 V and a FF of 71.4%, under the illumination of AM 1.5, 100 mW cm−2. In comparison, the PCE of the OSC based on BDT-3T(3-Hex)-ID as the donor is 1.06% under the same experimental conditions.

 Please wait while we load your content...
Please wait while we load your content...