Asymmetric N,N′-ethylene-bridged azole-based compounds: Two way control of the energetic properties of compounds†
Abstract
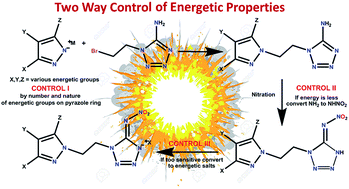
Reactions of various energetic pyrazole, triazole and tetrazole salts with 1-(2-bromoethyl)-5-aminotetrazole, in the presence of a phase transfer catalyst, resulted in new asymmetric N,N′-ethylene-bridged azole-based energetic compounds having diversified functionalities and properties. The availability of the aminotetrazole moiety for conversion to nitroimino(tetrazole) provides a route for further modifying energetic properties. All the compounds were thoroughly characterized by IR, NMR [1H, 13C{1H}, 15N], elemental analyses, and differential scanning calorimetry (DSC). Some were also further characterized using single-crystal X-ray diffraction studies. Impact and friction sensitivities were measured and heats of formation and detonation performances were calculated. Results show that combination of different energetic heterocycles broadens options for the design of desirable energetic compounds.

 Please wait while we load your content...
Please wait while we load your content...