Tailoring atomic distribution in micron-sized and spherical Li-rich layered oxides as cathode materials for advanced lithium-ion batteries†
Abstract
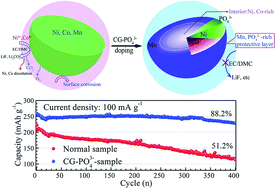
Li-rich layered oxides with large capacity are considered as one of the most promising cathode materials for the next generation lithium-ion batteries (LIBs). However, Li-rich layered oxides usually deliver unsatisfactory volumetric energy density, poor cycle life and inferior thermal stability. Here, a concentration-gradient doping strategy is introduced for the first time to meet the above challenges. Surprisingly, the atomic distribution in micron-sized and spherical Li-rich layered oxides is tailored after concentration-gradient PO43− polyanion doping, in which Ni and Co atoms decrease continually and Mn atoms increase gradually from the center to the surface in a single particle. As expected, the concentration-gradient PO43− doped oxides exhibit a high initial volumetric energy density of 2027 W h L−1, long cycle life with a capacity retention of 88.2% within 400 cycles, and enhanced thermal stability. These improved performances are believed to be attributed to the formation of the stable Mn-rich and PO43−-rich shell layer, which is beneficial to mitigate the interreaction between Ni4+/Co4+ and the electrolyte in the highly delithiated state and suppress the aggregation of primary grains during cycles. These results demonstrate the feasibility of manipulating atomic distribution by the innovative concentration-gradient doping means, which also provides new insights into desired cathode for LIBs.

 Please wait while we load your content...
Please wait while we load your content...