Alkali metals as efficient A-site acceptor dopants in proton conducting BaZrO3
Abstract
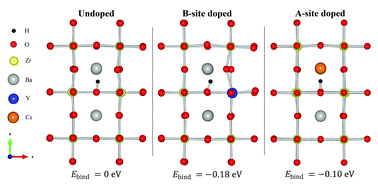
In the present contribution, we assess the efficiency of the alkali metals (Na, K, Rb and Cs) as A-site acceptor dopants in proton conducting BaZrO3 by first principles calculations. The calculated acceptor–proton complexes become weaker with increasing dopant size, with binding energies ranging from −0.33 eV for Na to −0.10 eV for Cs, which is in the range of, or even lower than, those found for B-site doped BaZrO3. By mapping out all relevant minimum energy pathways for the proton, we reveal that the highest migration energy barrier for most of the alkali metals is comparable or even lower than that of Y. Further, all A-site dopants display more exothermic hydration enthalpies compared to that of Y-doped BaZrO3, ranging from −131 kJ mol−1 to −83 kJ mol−1 for Na and Cs, respectively. The calculated dopant solubility increases in the order Na < Cs < Rb < K, with the predicted solubilities of the two latter being in the range of that of e.g. Y. Although Cs would lead to the highest proton mobility, the higher solubility of K and Rb renders them more attractive A-site dopants for BaZrO3. Overall, our results suggest that alkali metals as A-site dopants may enhance the bulk proton conductivity of BaZrO3, compared to Y-doped BaZrO3.

 Please wait while we load your content...
Please wait while we load your content...