A synergistic effect between layer surface configurations and K ions of potassium vanadate nanowires for enhanced energy storage performance†
Abstract
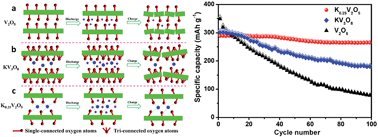
Layered metal vanadates, especially alkali metal vanadates, have been extensively studied in energy storage. Generally, vanadates exhibit more stable electrochemical performance than pristine vanadium oxides, and different vanadates also vary in the performance. However, the detailed mechanisms of the variation in the performance of vanadates and vanadium oxides are poorly explored. Here we choose and construct three typical layered vanadium-based nanowires (V2O5, KV3O8 and K0.25V2O5), and investigate the origin of the enhanced electrochemical performance of the potassium vanadates compared to V2O5, based on crystal structure analysis, electrochemical tests, ex situ ICP measurements and in situ XRD detections. We demonstrate a synergistic effect between layer surface configurations and K ions of potassium vanadate nanowires, which leads to the great improvement in electrochemical stability of K0.25V2O5. The layer surface configuration of K0.25V2O5 only consists of single-connected oxygen atoms, which provides strong interaction with the K ions. And the stabilized K ions act as “pillars” between interlayers to protect the layered structures from collapse in the charge/discharge process. This work provides a further insight into alkali metal vanadates, and benefits the design of ideal electrode materials in the energy storage field.


 Please wait while we load your content...
Please wait while we load your content...