ZIF-8 derived carbon (C-ZIF) as a bifunctional electron acceptor and HER cocatalyst for g-C3N4: construction of a metal-free, all carbon-based photocatalytic system for efficient hydrogen evolution†
Abstract
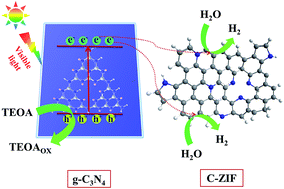
A ZIF-8 derived carbon (C-ZIF)/g-C3N4 composite was constructed for the first time through facile thermal condensation of a zeolitic imidazolate framework (ZIF-8) and melamine. The obtained C-ZIF/g-C3N4 composite exhibited an obviously enhanced photocatalytic H2 production rate compared to pure g-C3N4 under visible light irradiation. The 1 wt% C-ZIF/g-C3N4 composite without loading the Pt co-catalyst showed 36.2 times higher H2 evolution rate than that of pure g-C3N4, which is even 2.8 times higher than that of Pt/g-C3N4 (the state-of-the-art g-C3N4-based photocatalyst). It was revealed by photoluminescence spectroscopy, time-resolved fluorescence spectroscopy and electrochemical impedance spectroscopy that the formed C-ZIF and g-C3N4 junction could promote quick charge carrier separation and transfer. The C-ZIF not only acted as an effective electron acceptor, but also functioned as an efficient hydrogen evolution reaction (HER) cocatalyst to promote photocatalytic hydrogen evolution. Our work provides an effective way for the development of metal-free, all carbon-based photocatalysts for H2 evolution.


 Please wait while we load your content...
Please wait while we load your content...