Polydopamine nanotubes: bio-inspired synthesis, formaldehyde sensing properties and thermodynamic investigation
Abstract
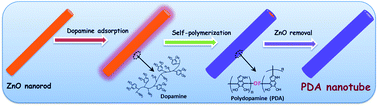
This work reports a bio-inspired synthesis of polydopamine (PDA) nanotubes with a desired wall thickness and their applications for trace-level formaldehyde sensing. To synthesize PDA nanotubes, batch prepared ZnO nanorods are selected as templates. Then, dopamine molecules with both catechol and amine groups are self-assembled onto the surface of ZnO nanorods like mussels adhered strongly on inorganic or organic substrates. After that, the adhered dopamine molecules are self-polymerized in alkaline solution to form polydopamine. Polydopamine nanotubes can be obtained by the removal of ZnO templates in NH4Cl aqueous solution. The characterization results indicate that the wall thicknesses of the PDA nanotubes are tunable in the range of 13–75 nm. To construct a micro-gravimetric gas sensor, PDA nanotubes are uniformly coated onto a quartz crystal microbalance (QCM). The sensing results show that the detection limit towards HCHO is lower than 100 ppb. Furthermore, the sensitivity can be enhanced by increasing the pore volume of PDA nanotubes. Compared with common PDA materials (like amorphous particles), PDA nanotubes exhibit improved formaldehyde sensing properties in terms of sensitivity, selectivity, and response/recovery speed. Based on the temperature-varying micro-gravimetric experiment, enthalpy change (ΔH°) is quantitatively obtained as −53.6 kJ mol−1, which indicates that the interaction between PDA nanotubes and HCHO molecules belongs to typical hydrogen-bond linking. This ΔH° value is also verified by the theoretical calculations of the binding energy by using Gaussian software.

 Please wait while we load your content...
Please wait while we load your content...