Moisture induced isotopic carbon dioxide trapping from ambient air†
Abstract
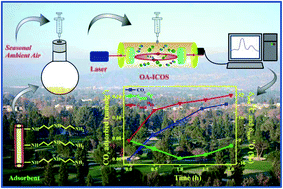
Clay based solid adsorbents comprised of several viable amines having varied amine densities and adsorption sites have been designed to capture isotopic CO2 from ambient air under standard temperature and pressure, keeping an eye on the preferential adsorption of the three major abundant isotopes of CO2 (12C16O2, 13C16O2, and 12C16O18O). Relative humidity induced isotopic CO2 adsorption, which appears to have received still little or even no attention, has been demonstrated using a laser based integrated cavity output spectroscopy technique and the adsorption kinetics describing the CO2 uptake rate were investigated to evaluate the efficiency of the adsorbents as well as to explore the underlying adsorption mechanism. In addition, all these novel adsorbents exhibit reversible CO2 adsorption in the oxidative environment and exceptional durability even after a prolonged cyclic adsorption–desorption study.

 Please wait while we load your content...
Please wait while we load your content...