High-capacity organic cathode active materials of 2,2′-bis-p-benzoquinone derivatives for rechargeable batteries†
Abstract
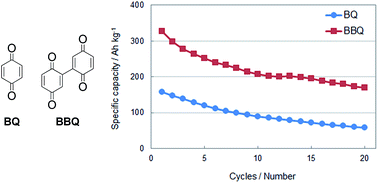
Rechargeable batteries using organic cathode materials are expected to afford high mass energy densities since these materials can undergo multiple electron redox reactions per molecule. Although the batteries using benzoquinone (BQ) derivatives as organic cathode active materials exhibited high theoretical capacity, their practical capacities and cycle retention were far from satisfactory. To overcome these problems, dimeric BQ derivatives based on the 2,2′-bis-p-benzoquinone (BBQ) framework were synthesized, and the charge–discharge behaviour of the prepared cells using BBQs as the cathode active materials was investigated. BBQ-based cells exhibited excellent performance compared to those based on BQ monomers. For example, the BBQ cell afforded a high initial capacity of 358 A h kg−1 (more than twice that of current lithium-ion batteries that use LiCoO2 as the cathode active material) and a high cycle retention of 198 A h kg−1 at 50 cycles. Electrochemical measurements and density functional theory (DFT) calculations indicated that three electron-redox reactions generally occur in BBQ derivatives, although (OMe)2-BBQ appeared to undergo a four-electron redox reaction.

 Please wait while we load your content...
Please wait while we load your content...