An electrochemical method to enhance the performance of metal oxides for photoelectrochemical water oxidation†
Abstract
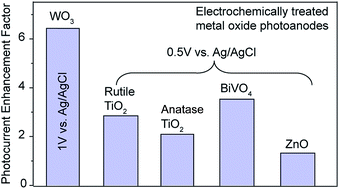
It has recently been demonstrated that the photoelectrochemical performance of a number of metal oxides can be substantially improved by controllably increasing their carrier densities through controlled introduction of defects such as oxygen vacancies. The creation of defects can be achieved via different synthetic methods, including hydrogenation, thermal treatment in oxygen deficient environment, chemical reductions, ion bombardment and electrochemical reductions. Here we report a general strategy to prepare oxygen-deficient metal oxides, including WO3, TiO2 (rutile and anatase), BiVO4, and ZnO, by electrochemically induced formation of low valent metal species. These electrochemically treated metal oxides show significantly enhanced photoactivity, as a result of improved charge injection and charge separation efficiency. The reported electrochemical method in this work represents a simple, rapid, highly scalable and safe approach to prepare high performance metal oxide photoanodes.
- This article is part of the themed collection: Water splitting and photocatalysis

 Please wait while we load your content...
Please wait while we load your content...