On the relationship between chemical expansion and hydration thermodynamics of proton conducting perovskites
Abstract
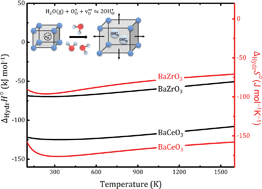
In this contribution, we determine the compositional dependence of the chemical expansion and entropy of hydration of the proton conducting perovskites BaZrO3, BaSnO3, BaCeO3, and SrZrO3 by first principles phonon calculations. The calculations reveal that the cubic BaZrO3 and BaSnO3, which display the least favourable hydration enthalpies, −72 and −65 kJ mol−1, respectively, exhibit the most favourable entropies, −108 and −132 J mol−1 K−1, respectively. The strong compositional dependency of the hydration entropy primarily originates from the entropy gain upon filling the oxygen vacancy, which is closely related to the chemical expansion coefficient of oxygen vacancies, and thus the chemical expansion upon hydration. The chemical expansion coefficient of oxygen vacancies is more negative for the cubic than the orthorhombic perovskites, leading to a considerably larger chemical expansion upon hydration of the former. The calculations therefore suggest that challenges associated with chemical expansion upon hydration of BaZrO3 proton conducting electrolytes to some extent can be avoided, or reduced, by partial substitution of Zr by Ce.

 Please wait while we load your content...
Please wait while we load your content...