MnO nanoparticles with cationic vacancies and discrepant crystallinity dispersed into porous carbon for Li-ion capacitors†
Abstract
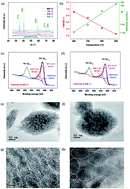
MnO nanoparticles with cationic vacancies and discrepant crystallinity were prepared through a one-step hydrothermal synthesis followed by calcination at different temperatures. Glucose was used as both a reducing agent to introduce cationic vacancies with a content of ∼5.5% into MnO nanocrystals, and a carbon source to encapsulate MnO nanocrystals in a three dimensional porous framework. Cationic vacancies benefit phase transition in a conversion reaction, and together with a low degree of crystallinity, may also provide more void spaces for ion diffusion (3.37 × 10−13 cm2 s−1). Three dimensional porous carbon with a pore volume of 0.27 cm3 g−1 demonstrated a high electrical conductivity of 6.25 S cm−1 and offered fast pathways for charge transfer and penetration of the electrolyte. Such a synergistic structure endowed MnO with excellent electrochemical properties including a considerably enhanced capacity of 650 mA h g−1 at a current density of 1000 mA g−1. Li ion capacitors based on such a MnO anode and activated carbon cathode achieved the maximum energy density of 220 W h kg−1, and the capacitance retention was 95.3% after 3600 cycles at a rate of 5000 mA g−1.

 Please wait while we load your content...
Please wait while we load your content...